Peptide Bonds
Peptide Bonds: The Foundation of Peptides and Proteins
The peptide bond is the fundamental chemical linkage that connects amino acids together to form peptides and proteins. Understanding the nature of the peptide bond is crucial for comprehending the structure, stability, and function of these vital biological macromolecules.
What is a Peptide Bond?
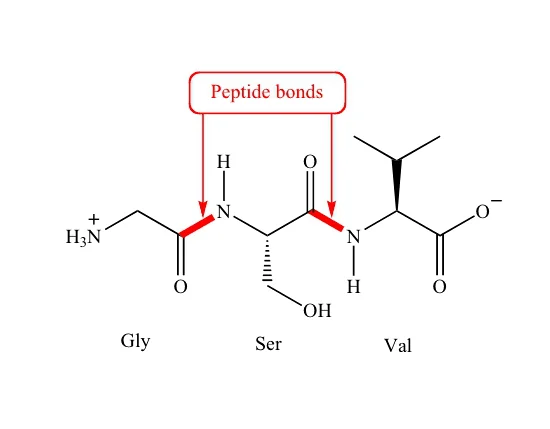
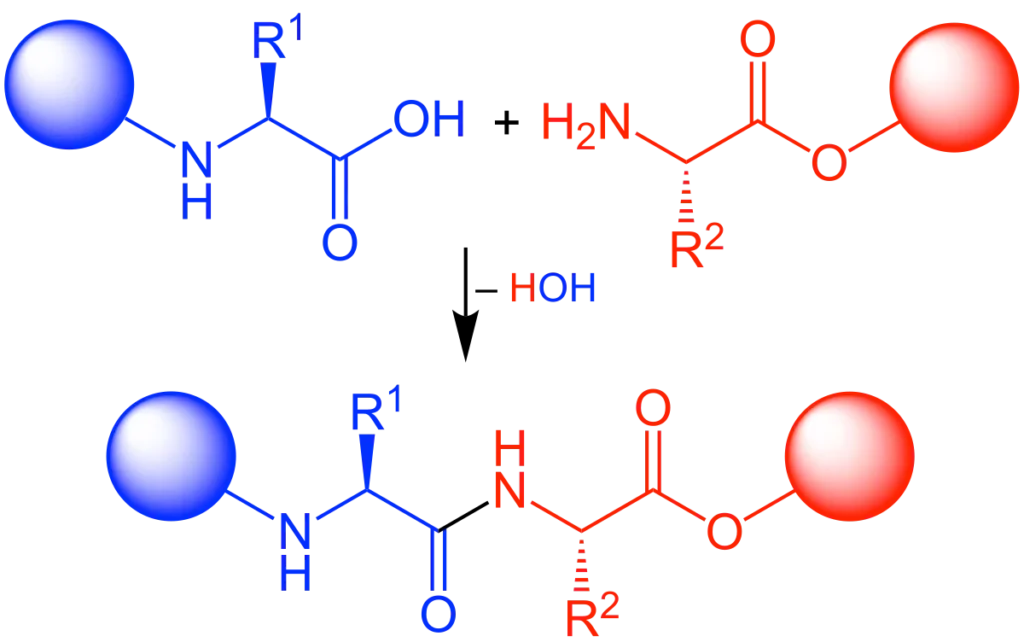
A peptide bond is an amide bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. This reaction is a dehydration synthesis (also known as a condensation reaction) because a molecule of water (H2O) is removed during its formation.
The reaction can be visualized as:
Amino Acid 1 (Carboxyl Group) + Amino Acid 2 (Amino Group) → Dipeptide + H2O
The resulting bond is a C-N bond, specifically between the carbonyl carbon (C=O) of one amino acid and the nitrogen atom of the next amino acid.
Characteristics of the Peptide Bond
The peptide bond possesses several unique characteristics that are critical for protein structure:
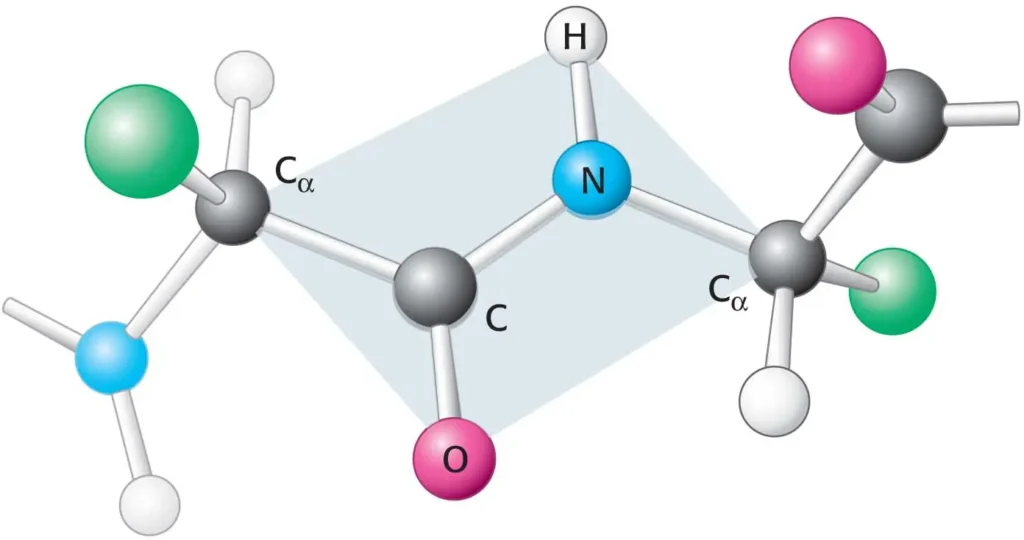
Planar Nature: The peptide bond (C-N) and the four atoms directly attached to it (the carbonyl oxygen, the carbonyl carbon, the amide nitrogen, and the amide hydrogen) lie in the same plane. This rigidity is due to the partial double-bond character of the C-N bond, resulting from resonance between the carbonyl group and the amide nitrogen.
Resonance: The electrons are delocalized across the C-O and C-N bonds, giving the C-N bond about 40% double-bond character. This prevents free rotation around the C-N bond.
Trans Configuration: In most peptide bonds, the alpha-carbons of adjacent amino acids are in a trans configuration relative to the peptide bond. This arrangement minimizes steric hindrance between the side chains of the amino acids. A cis configuration is rare but can occur, particularly with proline residues.
Polarity: The peptide bond is polar due to the electronegativity difference between oxygen, nitrogen, and carbon. The carbonyl oxygen has a partial negative charge, and the amide nitrogen has a partial positive charge. This polarity allows for hydrogen bonding, which is crucial for the formation of secondary protein structures like alpha-helices and beta-sheets.
Stability: Peptide bonds are remarkably stable under physiological conditions, resisting hydrolysis (breaking down by water). This stability is essential for maintaining the integrity of proteins within living organisms. However, they can be broken by strong acids or bases, or by specific enzymes called proteases (or peptidases).
Formation and Hydrolysis
Formation: Peptide bonds are formed during protein synthesis (translation) on ribosomes in cells, where amino acids are linked sequentially according to the genetic code. In laboratory settings, they are formed through chemical peptide synthesis methods like Solid-Phase Peptide Synthesis (SPPS).
Hydrolysis: The breaking of a peptide bond is called hydrolysis, which involves the addition of a water molecule. This process is catalyzed by proteases in biological systems (e.g., during digestion) or by harsh chemical conditions in the lab.
Significance in Peptides and Proteins
The formation of peptide bonds allows for the creation of long, diverse chains of amino acids. The sequence of these amino acids, linked by peptide bonds, defines the primary structure of a peptide or protein. The planar and rigid nature of the peptide bond, combined with the rotational freedom around the alpha-carbon bonds (phi and psi angles), dictates how the polypeptide chain can fold into its complex three-dimensional structures, which are essential for its biological function.
At Peptide Biologics, our synthesis processes are meticulously designed to ensure the correct and stable formation of peptide bonds, resulting in high-quality peptides that maintain their structural integrity and biological activity for your research needs.
